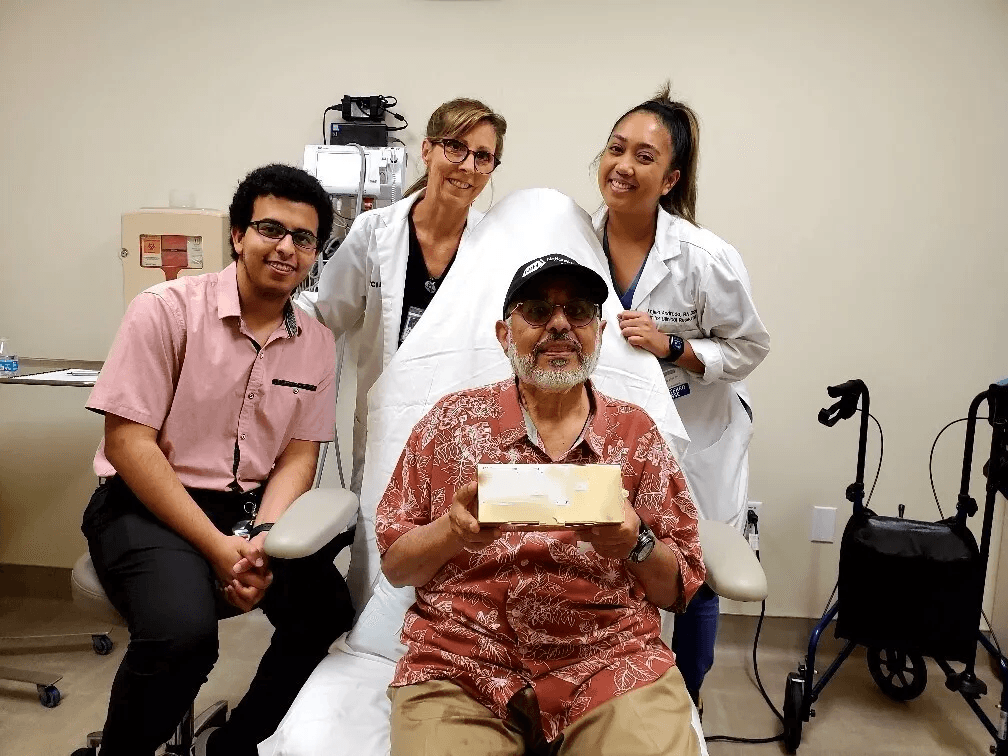
AnnJi Pharmaceutical Co., Ltd. and Avenue Therapeutics are excited to announce that Mike S. is the first Kennedy’s Disease patient to participate and be dosed in the clinical trial of AJ201 at the University of California, Irvine.
AnnJi launched a first-in-patient Phase 1/2a study of AJ201, a curcumin-based compound, to evaluate its safety, tolerability, and related biochemical and pharmacological activities in Kennedy’s Disease patients.
AJ201 is designed to activate the NRF1 and NRF2 cellular pathways to promote degradation of the mutant androgen receptor (AR) protein that is responsible for Kennedy’s Disease. AJ201 has been granted orphan drug status by the FDA and EMA for the treatment of Kennedy’s Disease. AJ201 has been granted orphan drug status by the FDA and EMA for the treatment of Kennedy’s Disease.
The study will be conducted at six sites in the United States in 2023. There are currently six AnnJi drug trial distribution sites, listed below.
Site 01: University of California, Irvine
Principal Investigator: Tahseen Mozaffar, MD
Contact: Pola Gaid, 714-456-6191
Site 02: National Institutes of Health
Principal Investigator: Christopher Grunseich, MD
Contact: Angela Kokkinis, 301-451-8146
Site 03: Stanford University
Principal Investigator: John W. Day, MD, PhD
Contact: Emily Lien
Site 04: Mayo Clinic Rochester
Principal Investigator: Eric J. Sorenson, MD
Contact: Jane Sultze, 507-538-5523
Site 05: Mayo Clinic Jacksonville
Principal Investigator: Bjorn E. Oskarsson, MD
Contact: Megan Donahue, 904-953-3647
Site 06: University of Washington in St. Louis
Principal Investigator: Alan Pestronk, MD
Contact: Oliver Doerr or Jennifer M. Koniak, 314-362-1626
Read the news release from AnnJi: Anji Biotech's New Drug AJ201 Has Achieved Impressive Clinical Phase 1b/2a Trial Results, Demonstrating Positive Therapeutic Efficacy and Potential.
Read the news release from Avenue Therapeutics: Avenue Therapeutics Announces First Patient Dosed in Phase 1b/2a Clinical Trial of AJ201 for the Treatment of Spinal and Bulbar Muscular Atrophy (Kennedy's Disease)
Learn more about AJ201 and AnnJi Pharmaceutical Co., Ltd.
Learn more about Avenue Therapeutics
Follow-Up News
Read the KDA News article: FDA Grants Fast Track Designation for AJ201
Read the KDA News article: AnnJi Showcases Promising Results for AJ201 in Kennedy’s Disease Clinical Trial


